Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
Self-Quiz Questions
|
|
| 1. | Choose the pair of terms that correctly completes this
sentence:
Catabolism is to anabolism as ____ is to ____. a. | exergonic;
spontaneous | b. | exergonic; endergonic | c. | free energy;
entropy | d. | work; energy | e. | entropy;
enthalpy | | |
|
|
| 2. | Most
cells cannot harness heat to perform work because a. | heat is not a form of energy. | b. | cells do not
have much heat; they are relatively cool. | c. | temperature is usually uniform throughout a
cell. | d. | heat can never be used to do work. | e. | heat denatures
enzymes. | | |
|
|
| 3. | According to the first law of thermodynamics, a. | matter can be
neither created nor destroyed. | b. | energy is conserved in all processes. | c. | all processes
increase the order of the universe. | d. | systems rich in energy are intrinsically
stable. | e. | the universe constantly loses energy because of
friction. | | |
|
|
| 4. | Which
of the following metabolic processes can occur without a net influx of energy from some other
process? a. | ADP +
Pi 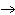 ATP +
H2O ATP +
H2O | b. | C6H12O6 + 6 O2 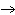 6 CO2 + 6
H2O 6 CO2 + 6
H2O | c. | 6 CO2 + 6 H2O 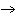 C6H12O6 + 6
O2 C6H12O6 + 6
O2 | d. | amino acids 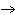 protein
protein | e. | glucose + fructose 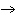 sucrose
sucrose | | |
|
|
| 5. | If an
enzyme has been inhibited noncompetitively, a. | the  G for the reaction it catalyzes will always be negative. G for the reaction it catalyzes will always be negative. | b. | the active site
will be occupied by the inhibitor molecule. | c. | raising
substrate concentration will increase the inhibition. | d. | more energy will
be necessary to initiate the reaction. | e. | the inhibitor molecule may be chemically unrelated to the
substrate. | | |
|
|
| 6. | If an
enzyme solution is saturated with substrate, the most effective way to obtain an even faster yield of
products is to a. | add more of the
enzyme. | b. | heat the solution to 90°C. | c. | add more
substrate. | d. | add an allosteric inhibitor. | e. | add a
noncompetitive inhibitor. | | |
|
|
| 7. | If an
enzyme is added to a solution where its substrates and products are in equilibrium, what would
occur? a. | Additional
product would be formed. | b. | Additional substrate would be formed. | c. | The reaction
would change from endergonic to exergonic. | d. | The free energy of the system would
change. | e. | Nothing; the reaction would stay at
equilibrium. | | |
|
|
| 8. | Some
bacteria are metabolically active in hot springs because a. | they are able to
maintain a cooler internal temperature. | b. | high temperatures make catalysis
unnecessary. | c. | their enzymes have high optimal
temperatures. | d. | their enzymes are insensitive to
temperature. | e. | they use molecules other than proteins as their main
catalysts. | | |
|
|
| 9. | Which
of the following characteristics is not associated with allosteric regulation of an enzyme's
activity? a. | A mimic of the
substrate competes for the active site. | b. | A naturally occurring molecule stabilizes a catalytically
active conformation. | c. | Regulatory molecules bind to a site remote from the active
site. | d. | Inhibitors and activators may compete with one
another. | e. | The enzyme usually has a quaternary
structure. | | |
|
|
| 10. | In
the following branched metabolic pathway, a dotted arrow with a minus sign symbolizes inhibition of a
metabolic step by an end product:
Which reaction would prevail if both Q and S were present in the cell
in high concentrations? a. | L 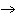 M
M | b. | M 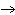 O O | c. | L 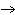 N N | d. | O  P P | e. | R  S S | | |
|